MULTAQ is a prescription medicine used to lower the chance of hospitalization for atrial fibrillation (AFib) in people who currently have a normal heart rhythm and have had certain types of AFib (paroxysmal or persistent AFib) in the past. It is not known if MULTAQ is safe and effective in children younger than age 18 years old.
.png 375w)
MULTAQ can help
you stay IN normal
heart rhythm & OUT
of the hospital
MULTAQ can help you stay IN normal heart rhythm & OUT of the hospital
MULTAQ is an FDA-approved heart rhythm medication for
people with a normal heart rhythm and a history of
paroxysmal or persistent AFib.

In two 12-month clinical studies, people who took MULTAQ had:
A lower chance of an AFib event

MULTAQ lowered the chance of an AFib event by 25%
64.1% of patients on MULTAQ had an AFib event at 1 year compared to 75.2% of patients on placebo.
Please see EURIDIS/ADONIS Study design below.
In two 12-month clinical studies, people who took MULTAQ had:
A normal heart rhythm for twice as long as patients taking placebo
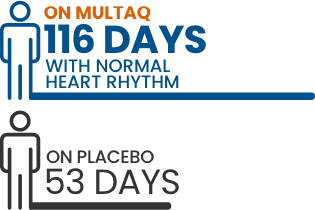.png 315w)
Please see EURIDIS/ADONIS Study design below.

In another 12-month clinical study, people who took MULTAQ had:
A lower chance of heart-related hospitalizations or death

MULTAQ lowered the chance of either heart-related hospitalizations or death by 24% vs placebo
Result is based only on reduction of heart-related hospitalizations. 31.6% of patients on MULTAQ had either heart-related hospitalization or death compared to 39.2% of patients on placebo.
Please see ATHENA study design below.
EURIDIS/ADONIS Study Design: Two similar clinical studies that assessed the efficacy and safety of MULTAQ in 1237 patients in normal sinus rhythm with a history of paroxysmal or persistent atrial fibrillation (AFib) or atrial flutter (AFL). 828 patients were given 400 mg of MULTAQ twice daily and 409 patients were given placebo twice daily for 12 months. The study compared the amount of time heart rhythm remained normal (or the time to first recurrence of AFib/AFL) within 12 months for MULTAQ and placebo.
ATHENA Study Design: A clinical study that assessed the efficacy and safety of MULTAQ in 4628 patients in normal sinus rhythm with a history of paroxysmal or persistent AFib or AFL. Patients were 70 years of age or older and had at least one other condition of the heart or blood vessels in addition to their AFib or AFL. 2301 patients were given 400 mg of MULTAQ twice daily and 2327 patients were given placebo twice daily for up to 30 months, with a median* follow-up of 22 months. The study compared the chance of either first heart or blood vessel-related hospitalization or death for MULTAQ and placebo.
*Median is the middle number in a group of numbers arranged from lowest to highest.
Learn about potential side effects of MULTAQ
More facts about MULTAQ
More than 900,000 people with paroxysmal or persistent AFib have been prescribed MULTAQ since its FDA approval more than 15 years ago.
MULTAQ has been studied in multiple clinical trials totaling nearly 6000 people with paroxysmal or persistent AFib.
Need to save on your prescription for MULTAQ?
WHAT IS MULTAQ?
MULTAQ is a prescription medicine used to lower the chance of hospitalization for atrial fibrillation (AFib) in people who currently have a normal heart rhythm and have had certain types of AFib (paroxysmal or persistent AFib) in the past. It is not known if MULTAQ is safe and effective in children younger than age 18 years old.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about MULTAQ?
MULTAQ may cause serious side effects, including:
-
Increased risk of death, stroke, and heart failure in people with:
-
A certain type of heart failure called decompensated heart failure. Heart failure is when your heart does not pump blood through your body as well as it should. MULTAQ can cause new or worsening heart failure.
Do not take MULTAQ if you have symptoms of heart failure that recently worsened and you were hospitalized, or if you have severe heart failure. Call your healthcare provider right away if you develop any of the following signs or symptoms of heart failure during treatment with MULTAQ: shortness of breath or wheezing at rest; wheezing, chest tightness, or coughing up frothy sputum at rest, nighttime, or after minor exercise; trouble sleeping or waking up at night because of breathing problems; using more pillows to prop yourself up at night so you can breathe more easily; gaining more than 5 pounds quickly; increasing swelling of feet or legs - A certain type of irregular heartbeat (rhythm) called permanent atrial fibrillation (AFib). Permanent AFib is when you and your healthcare provider decide not to try to change your heart rhythm back to a normal heart rhythm or your heart rhythm cannot be changed back to a normal rhythm.
Do not take MULTAQ if you have permanent AFib. Your healthcare provider should check your heart rhythm regularly to make sure your heart keeps a normal rhythm.
Call your healthcare provider right away if you develop any of the following signs or symptoms of AFib during treatment with MULTAQ such as: fast or irregular heartbeat or pulse; chest pain; dizziness or lightheadedness; tiredness or weakness; reduced ability to exercise; shortness of breath
-
MULTAQ doubles your risk of dying if you have these conditions. Your healthcare provider may give you a medicine to help prevent blood clots and decrease your risk of stroke during treatment with MULTAQ. Tell your healthcare provider right away if you develop any of the following signs or symptoms of stroke during treatment with MULTAQ such as: numbness or weakness in the face, arms, or legs, especially on 1 side of the body; confusion, trouble speaking, or difficulty understanding things; trouble seeing in 1 or both eyes; trouble walking, dizziness, loss of balance, or lack of coordination.
-
Liver problems. MULTAQ may cause severe liver problems, including life-threatening liver failure. Do not take MULTAQ if you have severe liver problems. Your healthcare provider may order blood tests to check your liver before you start taking MULTAQ and during treatment. Call your healthcare provider right away if you develop any of the following signs and symptoms of liver problems during treatment with MULTAQ: loss of appetite, nausea, vomiting; fever, feeling unwell, unusual tiredness; itching; yellowing of the skin or the whites of the eyes (jaundice); unusual darkening of the urine; right upper stomach area pain or discomfort
Who should not take MULTAQ?
See “What is the most important information I should know about taking MULTAQ?”
Do not take MULTAQ if:
-
you have a certain type of heart problem called heart block, and you do not have an implanted pacemaker
-
your heart rate is less than 50 beats each minute
-
you have had liver or lung problems after using amiodarone
-
you have a certain type of electrocardiogram (ECG) abnormality including QTc or PR interval prolongation
-
you take certain medicines that can change the amount of MULTAQ that gets into your body such as: nefazodone; ritonavir; ketoconazole; itraconazole; erythromycin; voriconazole; telithromycin; clarithromycin; cyclosporin
-
you take certain medicines that can lead to a dangerous abnormal heart rhythm such as: phenothiazines; tricyclic antidepressants; macrolide antibiotics; certain medicines for abnormal heart rhythm or fast heartbeat (Class I and III antiarrhythmics)
-
you are allergic to dronedarone or any of the other ingredients in MULTAQ
What should I tell my healthcare provider before taking MULTAQ?
Before taking MULTAQ, tell your healthcare provider about all of your medical conditions, including if you:
-
have any other heart problems, including heart rhythm problems, or have had a stroke
-
have an implanted pacemaker
-
have liver or kidney problems
-
have lung problems
-
have low levels of potassium or magnesium in your blood
-
are pregnant or plan to become pregnant. MULTAQ may harm your unborn baby
Females who can become pregnant-
Your healthcare provider will do a pregnancy test before you start treatment with MULTAQ
-
Use effective birth control (contraception) during treatment and for 5 days after your final dose of MULTAQ
-
Tell your healthcare provider right away if you think you are pregnant or become pregnant during treatment with MULTAQ
-
-
are breastfeeding or plan to breastfeed. It is not known if MULTAQ passes into your breast milk. Do not breastfeed during treatment and for 5 days after the final dose of MULTAQ
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking MULTAQ with certain other medicines may affect the amount of MULTAQ or other medicines in your blood and may increase your risk of side effects or affect how well MULTAQ or the other medicines work.
Especially tell your healthcare provider if you take: medicine for high blood pressure, chest pain, or other heart conditions; statin medicine to lower blood cholesterol; medicine for tuberculosis (TB); medicine for seizures; digoxin; warfarin or other blood thinner medicines; medicine for organ transplant; an herbal supplement called St. John’s wort; water pills (diuretics).
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
What should I avoid while taking MULTAQ?
Do not drink grapefruit juice during treatment with MULTAQ. Grapefruit juice can increase the amount of MULTAQ in your blood and can increase your chance of getting side effects.
What are the possible side effects of MULTAQ?
MULTAQ may cause serious side effects, including:
-
See “What is the most important information I should know about MULTAQ?”
-
Inflammation of the lungs, including scarring and thickening. Call your healthcare provider if you develop shortness of breath or a dry cough during treatment with MULTAQ
-
Low potassium and magnesium levels in your blood. This can happen if you take certain water pills (diuretics) during treatment with MULTAQ. Your healthcare provider may check you for this problem before and during treatment. Tell your healthcare provider if you develop any of the following symptoms of low potassium or low magnesium during treatment with MULTAQ: nausea or vomiting; weakness or sleepiness; muscle weakness, spasms, or tremors; loss of appetite; constipation; heart palpitations; tingling or numbness
-
Changes in the electrical activity in your heart called QT interval prolongation. QT interval prolongation can increase your chance of getting dangerous abnormal heart rhythms
-
Kidney problems and kidney failure. MULTAQ can cause changes in kidney function that can be serious and lead to kidney failure, especially in people with heart failure or people with low body fluid levels. Your healthcare provider will check your blood for signs of kidney problems during treatment. Tell your healthcare provider if you develop any of the following symptoms of kidney problems during treatment with MULTAQ: loss of appetite; nausea and vomiting; muscle cramps; dry, itchy skin; swelling of the feet and ankles; shortness of breath; trouble sleeping; urinating too much or too little
The most common side effects of MULTAQ include: diarrhea; weakness, lack of energy, and feeling very tired or sleepy (asthenia); nausea; skin problems such as redness, rash, and itching; stomach area (abdominal) pain; slow heart rate (bradycardia); vomiting; indigestion.
Your healthcare provider may stop treatment with MULTAQ if you develop certain side effects. These are not all of the possible side effects of MULTAQ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please click here for full Prescribing Information including Risk of SERIOUS SIDE EFFECTS.
Please click here for the MULTAQ Medication Guide (English).
If you are a patient experiencing problems with a Sanofi U.S. product, please contact Sanofi U.S. at 1-800-633-16101-800-633-1610.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
Sanofi does not provide medical advice, diagnosis, or treatment. The health information contained herein is provided for general educational purposes only. Your healthcare professional is the best source of information regarding your health. Please consult your healthcare professional if you have any questions about your health or treatment.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about MULTAQ?
MULTAQ may cause serious side effects, including:
-
Increased risk of death, stroke, and heart failure in people with:
-
A certain type of heart failure called decompensated heart failure. Heart failure is when your heart does not pump blood through your body as well as it should. MULTAQ can cause new or worsening heart failure.
Do not take MULTAQ if you have symptoms of heart failure that recently worsened and you were hospitalized, or if you have severe heart failure. Call your healthcare provider right away if you develop any of the following signs or symptoms of heart failure during treatment with MULTAQ: shortness of breath or wheezing at rest; wheezing, chest tightness, or coughing up frothy sputum at rest, nighttime, or after minor exercise; trouble sleeping or waking up at night because of breathing problems; using more pillows to prop yourself up at night so you can breathe more easily; gaining more than 5 pounds quickly; increasing swelling of feet or legs - A certain type of irregular heartbeat (rhythm) called permanent atrial fibrillation (AFib). Permanent AFib is when you and your healthcare provider decide not to try to change your heart rhythm back to a normal heart rhythm or your heart rhythm cannot be changed back to a normal rhythm.
Do not take MULTAQ if you have permanent AFib. Your healthcare provider should check your heart rhythm regularly to make sure your heart keeps a normal rhythm.
Call your healthcare provider right away if you develop any of the following signs or symptoms of AFib during treatment with MULTAQ such as: fast or irregular heartbeat or pulse; chest pain; dizziness or lightheadedness; tiredness or weakness; reduced ability to exercise; shortness of breath
-
MULTAQ doubles your risk of dying if you have these conditions. Your healthcare provider may give you a medicine to help prevent blood clots and decrease your risk of stroke during treatment with MULTAQ. Tell your healthcare provider right away if you develop any of the following signs or symptoms of stroke during treatment with MULTAQ such as: numbness or weakness in the face, arms, or legs, especially on 1 side of the body; confusion, trouble speaking, or difficulty understanding things; trouble seeing in 1 or both eyes; trouble walking, dizziness, loss of balance, or lack of coordination.
-
Liver problems. MULTAQ may cause severe liver problems, including life-threatening liver failure. Do not take MULTAQ if you have severe liver problems. Your healthcare provider may order blood tests to check your liver before you start taking MULTAQ and during treatment. Call your healthcare provider right away if you develop any of the following signs and symptoms of liver problems during treatment with MULTAQ: loss of appetite, nausea, vomiting; fever, feeling unwell, unusual tiredness; itching; yellowing of the skin or the whites of the eyes (jaundice); unusual darkening of the urine; right upper stomach area pain or discomfort
Who should not take MULTAQ?
See “What is the most important information I should know about taking MULTAQ?”
Do not take MULTAQ if:
-
you have a certain type of heart problem called heart block, and you do not have an implanted pacemaker
-
your heart rate is less than 50 beats each minute
-
you have had liver or lung problems after using amiodarone
-
you have a certain type of electrocardiogram (ECG) abnormality including QTc or PR interval prolongation
-
you take certain medicines that can change the amount of MULTAQ that gets into your body such as: nefazodone; ritonavir; ketoconazole; itraconazole; erythromycin; voriconazole; telithromycin; clarithromycin; cyclosporin
-
you take certain medicines that can lead to a dangerous abnormal heart rhythm such as: phenothiazines; tricyclic antidepressants; macrolide antibiotics; certain medicines for abnormal heart rhythm or fast heartbeat (Class I and III antiarrhythmics)
-
you are allergic to dronedarone or any of the other ingredients in MULTAQ
What should I tell my healthcare provider before taking MULTAQ?
Before taking MULTAQ, tell your healthcare provider about all of your medical conditions, including if you:
-
have any other heart problems, including heart rhythm problems, or have had a stroke
-
have an implanted pacemaker
-
have liver or kidney problems
-
have lung problems
-
have low levels of potassium or magnesium in your blood
-
are pregnant or plan to become pregnant. MULTAQ may harm your unborn baby
Females who can become pregnant-
Your healthcare provider will do a pregnancy test before you start treatment with MULTAQ
-
Use effective birth control (contraception) during treatment and for 5 days after your final dose of MULTAQ
-
Tell your healthcare provider right away if you think you are pregnant or become pregnant during treatment with MULTAQ
-
-
are breastfeeding or plan to breastfeed. It is not known if MULTAQ passes into your breast milk. Do not breastfeed during treatment and for 5 days after the final dose of MULTAQ
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking MULTAQ with certain other medicines may affect the amount of MULTAQ or other medicines in your blood and may increase your risk of side effects or affect how well MULTAQ or the other medicines work.
Especially tell your healthcare provider if you take: medicine for high blood pressure, chest pain, or other heart conditions; statin medicine to lower blood cholesterol; medicine for tuberculosis (TB); medicine for seizures; digoxin; warfarin or other blood thinner medicines; medicine for organ transplant; an herbal supplement called St. John’s wort; water pills (diuretics).
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
What should I avoid while taking MULTAQ?
Do not drink grapefruit juice during treatment with MULTAQ. Grapefruit juice can increase the amount of MULTAQ in your blood and can increase your chance of getting side effects.
What are the possible side effects of MULTAQ?
MULTAQ may cause serious side effects, including:
-
See “What is the most important information I should know about MULTAQ?”
-
Inflammation of the lungs, including scarring and thickening. Call your healthcare provider if you develop shortness of breath or a dry cough during treatment with MULTAQ
-
Low potassium and magnesium levels in your blood. This can happen if you take certain water pills (diuretics) during treatment with MULTAQ. Your healthcare provider may check you for this problem before and during treatment. Tell your healthcare provider if you develop any of the following symptoms of low potassium or low magnesium during treatment with MULTAQ: nausea or vomiting; weakness or sleepiness; muscle weakness, spasms, or tremors; loss of appetite; constipation; heart palpitations; tingling or numbness
-
Changes in the electrical activity in your heart called QT interval prolongation. QT interval prolongation can increase your chance of getting dangerous abnormal heart rhythms
-
Kidney problems and kidney failure. MULTAQ can cause changes in kidney function that can be serious and lead to kidney failure, especially in people with heart failure or people with low body fluid levels. Your healthcare provider will check your blood for signs of kidney problems during treatment. Tell your healthcare provider if you develop any of the following symptoms of kidney problems during treatment with MULTAQ: loss of appetite; nausea and vomiting; muscle cramps; dry, itchy skin; swelling of the feet and ankles; shortness of breath; trouble sleeping; urinating too much or too little
The most common side effects of MULTAQ include: diarrhea; weakness, lack of energy, and feeling very tired or sleepy (asthenia); nausea; skin problems such as redness, rash, and itching; stomach area (abdominal) pain; slow heart rate (bradycardia); vomiting; indigestion.
Your healthcare provider may stop treatment with MULTAQ if you develop certain side effects. These are not all of the possible side effects of MULTAQ.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please click here for full Prescribing Information including Risk of SERIOUS SIDE EFFECTS.
Please click here for the MULTAQ Medication Guide (English).
If you are a patient experiencing problems with a Sanofi U.S. product, please contact Sanofi U.S. at 1-800-633-16101-800-633-1610.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
Sanofi does not provide medical advice, diagnosis, or treatment. The health information contained herein is provided for general educational purposes only. Your healthcare professional is the best source of information regarding your health. Please consult your healthcare professional if you have any questions about your health or treatment.

